🚀 PolyPid (NASDAQ: $PYPD) just completed enrollment in its pivotal Phase 3 trial with FDA Fast-Track status;
🚀 Targeting the massive $10B surgical infection market ;
🚀 Wall Street analysts project 300%+ potential return as trial results approach

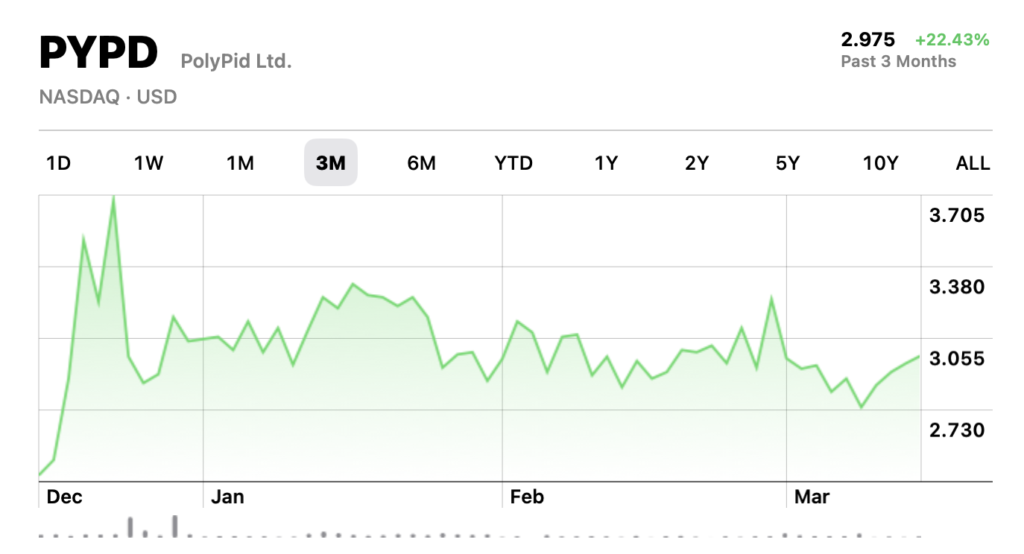
PolyPid (Nasdaq: PYPD), an under-the-radar medical innovator trading at just $2.74 per share, just announced a significant achievement with the successful completion of enrollment in its pivotal SHIELD II Phase 3 trial. The 800-patient study evaluates D-PLEX100, the company’s lead product candidate, for preventing infections after colorectal surgery—a critical healthcare challenge affecting millions of patients annually.
Surgical site infections (SSIs) represent a massive market opportunity estimated at over $10 billion in the US and EU combined, with up to 30% of colorectal surgeries developing infections despite current preventive measures.
This key milestone follows an independent Data Safety Monitoring Board’s highly positive recommendation after reviewing unblinded efficacy data from the first 430 enrolled patients. The board specifically recommended concluding the study at just 800 patients—the lowest sample size reassessment option available—which many analysts interpret as a potential signal of compelling efficacy trends.
According to the announcement, PolyPid is now on track to report top-line results by the end of Q2 2025, with plans to rapidly advance toward FDA submission leveraging coveted Fast Track and Breakthrough Therapy designations, which could significantly accelerate the approval timeline.
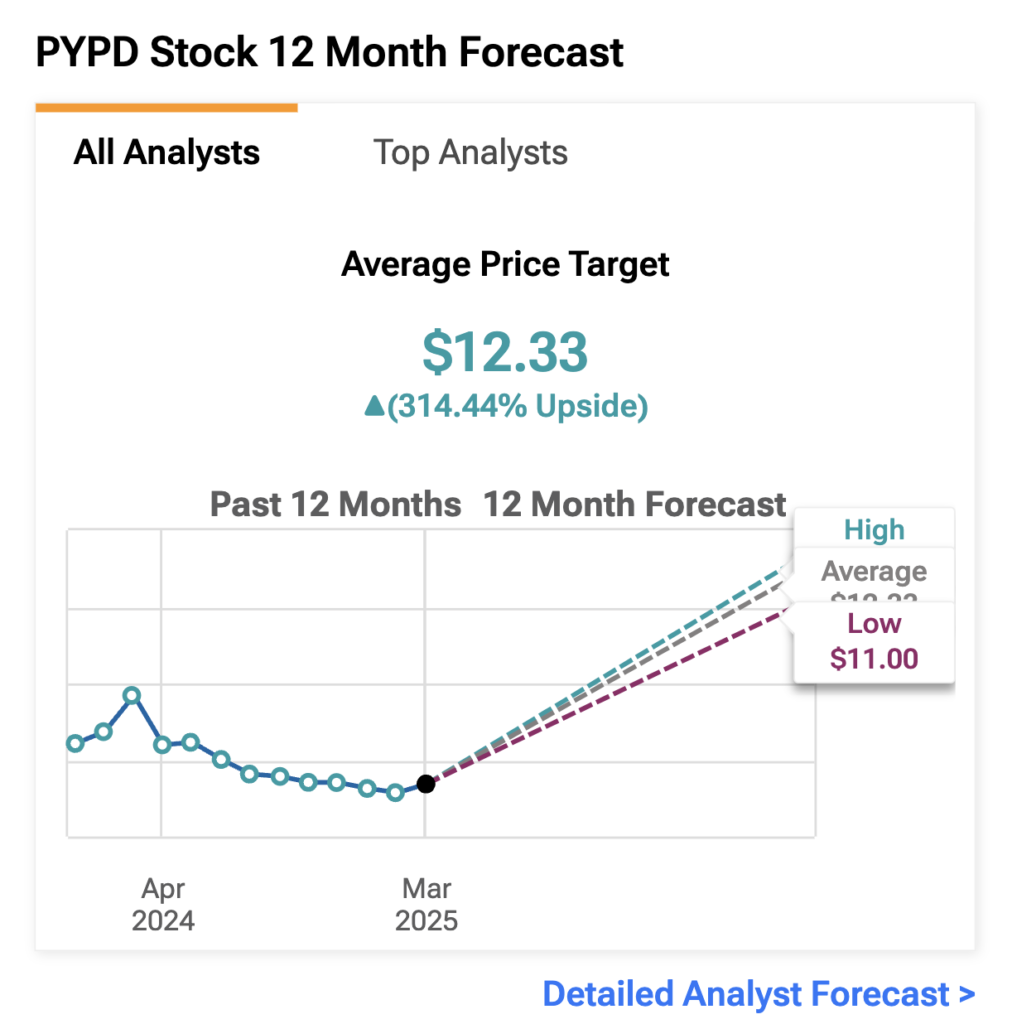
According to TipRanks, Wall Street analysts seem to maintain bullish outlooks on the small-cap biotech, with an average price target of $12.33 —representing massive+300% potential significant upside from current trading levels around $2.95.
The company’s recent $41 million financing deal provides substantial runway to reach these critical milestones without additional fundraising. This financial stability comes at a perfect time as the company approaches what could be a transformative catalyst in just a few months.
What makes D-PLEX100 potentially revolutionary? Unlike traditional antibiotics that work for just hours, PolyPid’s technology creates a “time-release” antibiotic shield that protects surgical sites for a full month. This extended protection addresses a major gap in current surgical infection prevention that could save healthcare systems billions in complication costs.
“We are excited to reach another critical milestone in our ongoing SHIELD II Phase 3 trial, reinforcing the positive trajectory of D-PLEX100’s development,” said Dikla Czaczkes Akselbrad, PolyPid’s Chief Executive Officer. “Importantly, we remain in active discussions with multiple potential partners for the commercialization of D-PLEX100 in various regions, starting with the U.S.”
For those looking for innovative companies that could be before major catalyst explosions, PolyPid represents a compelling case study. With FDA Fast Track and Breakthrough Therapy designations already secured, strong analyst support, and potentially market-moving trial results expected in the coming months, the stock could be the next multi-bagger that is still mostly under the radar, although that could change any minute.
Beyond its lead program, PolyPid is also developing applications of its drug delivery platform for cancer treatment and recently announced a collaboration with ImmunoGenesis targeting solid tumors, potentially expanding the market opportunity further.
With enrollment now complete in this pivotal trial, all eyes will be on the upcoming data readout expected before the end of Q2 2025. For investors willing to take on the inherent risks of biotech investing, the next few months could determine whether PolyPid remains an under-the-radar opportunity or emerges as one of 2025’s biotech success stories.
Click here to Read PolyPid’s full announcement, titled: “PolyPid Announces Successful Completion of Enrollment in Phase 3 SHIELD II Trial of D-PLEX100 for the Prevention of Abdominal Colorectal Surgical Site Infections”
PolyPid – Still under the Radar?
DISCLAIMER: Nothing in this report constitutes financial or investment advice, nor does it represent an offer to buy or sell securities. This report is published by Wall Street Wire. The operators of Wall Street Wire are not registered brokers, dealers, or investment advisers. This report is paid promotional and sponsored content related to PolyPid and was produced as part of their paid subscription to Wall Street Wire distribution and promotional content platform. This report was not reviewed by PolyPid prior to publication. Please review the full disclaimers and compensation details here: redditwire.com/terms. We are not responsible for the price targets mentioned in this article nor do we it endorse them, they are quoted based on publicly available news reports. Readers are advised to refer to the full reports mentioned on various systems and the disclaimers/disclosures they may be subject to.
