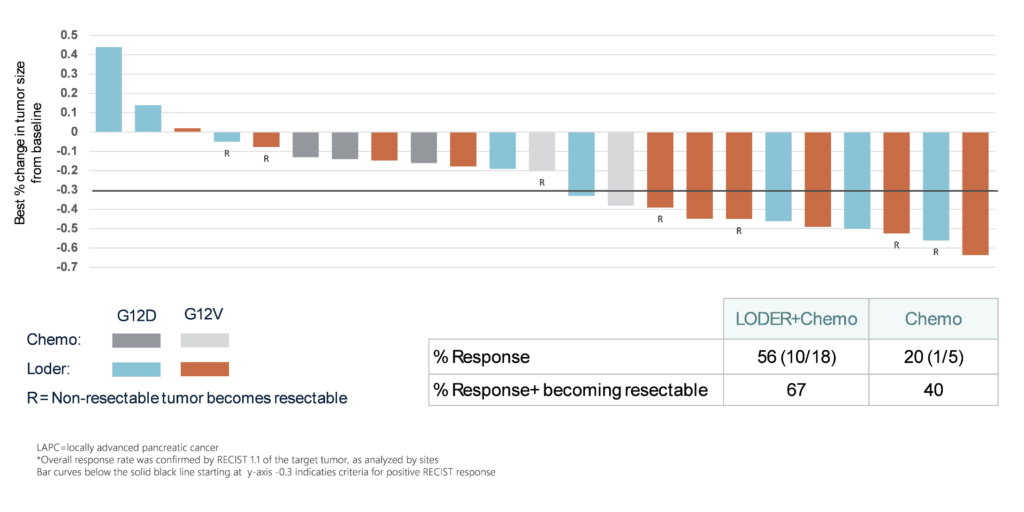
Silexion Therapeutics (NASDAQ: SLXN), a biotech company focused on innovative RNA interference (RNAi) therapies, has announced promising new data from its Phase 2 trial of LODER™, targeting patients with non-resectable locally advanced pancreatic cancer (LAPC). The data highlights a 56% objective response rate (ORR) and a 67% increase in tumor resectability, marking a significant advancement in treating a cancer type that has long been challenging to manage effectively. Beyond pancreatic cancer, these findings could have broader implications for a range of KRAS-driven cancers.
A Potential Shift in Pancreatic Cancer Treatment
Pancreatic cancer remains one of the most aggressive and difficult-to-treat cancers, often diagnosed at an advanced stage when therapeutic options are limited. Many patients are ineligible for surgery due to the tumor’s location or extent of spread. The new data from Silexion’s Phase 2 study indicates that LODER™ could alter this paradigm by converting previously inoperable tumors into candidates for surgery.
The trial focused on patients with KRAS G12D or G12V mutations, which are present in roughly 70% of pancreatic cancer cases. Traditional therapies have struggled to target these mutations effectively. LODER™, however, delivers small interfering RNA (siRNA) directly to the tumor, silencing the KRAS gene and preventing the production of proteins that promote cancer growth. This method addresses the root genetic cause and minimizes harm to surrounding healthy tissue.
Extending the Impact Beyond Pancreatic Cancer
KRAS mutations are not exclusive to pancreatic cancer; they are also significant in other aggressive cancers, including lung, colorectal, and ovarian cancers. KRAS is one of the most commonly mutated oncogenes in human cancers, especially solid tumors, and is often linked to resistance to standard treatments.
Silexion’s next-generation product, SIL-204, aims to target a wider spectrum of KRAS mutations, beyond those found in pancreatic cancer. This expanded focus could potentially benefit many patients with limited treatment options for KRAS-driven cancers.
Detailed Results from the Clinical Trial
The Phase 2 trial enrolled 48 patients in the U.S. and Israel with locally advanced pancreatic cancer that was inoperable or marginally operable. The study was divided into two groups. The first group, consisting of 29 patients, received LODER™ in combination with standard chemotherapy, or chemotherapy alone, to compare overall survival rates. The second group, with 19 patients, assessed the effectiveness and safety of the treatment in those whose tumors were initially inoperable.
The results were encouraging: 56% of patients with the targeted KRAS mutations responded positively to the treatment. Notably, 67% of tumors deemed inoperable shrank sufficiently to become candidates for surgery, a critical outcome since surgery is often the best option for long-term survival in pancreatic cancer cases.
“We are excited by these findings, which demonstrate LODER’s potential to significantly increase tumor resectability in patients with non-resectable pancreatic cancer,” said Ilan Hadar, Chairman and CEO of Silexion. “This data further validates our oncogene silencing strategy as we continue to expand our pipeline targeting KRAS-driven cancers.”
Advancing the Pipeline with SIL-204
Building on LODER’s success, Silexion is also advancing SIL-204, a next-generation siRNA therapy designed to target a broader range of KRAS mutations, including those currently beyond the reach of existing treatments. Preclinical studies show promising results for SIL-204, with improved stability and enhanced delivery, potentially making it more effective at silencing the KRAS gene.
The company plans to initiate Phase 2/3 clinical trials for SIL-204 in 2025-2026, targeting not only pancreatic cancer but also other cancers driven by KRAS mutations. Given the high prevalence of these mutations in numerous aggressive cancers, SIL-204 could represent a significant advancement in precision oncology, offering new treatment possibilities for patients with limited options.
Looking Forward: A Targeted Approach to Cancer Therapy
Silexion’s RNAi technology directly addresses the genetic drivers of cancer, rather than merely treating the disease’s symptoms. By delivering treatment directly to the tumor site and silencing the KRAS gene at its source, this approach has the potential to overcome long-standing barriers in the treatment of various KRAS-driven cancers.
As Silexion progresses through clinical trials and further explores the potential of its RNAi technology, it is poised to become a key player in the future of precision oncology. The recent Phase 2 data provide a promising indication that the company’s innovative strategies may offer new hope to patients battling some of the most challenging forms of cancer.
This article is for informational purposes only and is not intended to serve as financial, investment or any form of professional advice, recommendation or endorsement. Please review the full documentation detailing financial compensation disclosures and disclaimers the article is subject to. The Author, Global Markets News Network, is a commercial digital brand compensated to provide coverage of news and developments of the company aforementioned as detailed in the full documentation and it is thus subject to conflicts of interest.

